Hydrogen fuel, which can be obtained from fuels such as natural gas, methanol, or petroleum, and oxygen from the air electrochemically combine in the fuel cell to produce electricity. Heat and pure water vapor are the only by-products from the fuel cell's electrochemical reaction.
 |
. |
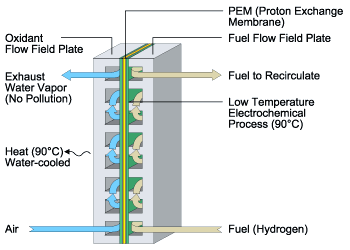
| Above: A schematic of the Proton Exchange Membrane (PEM) Fuel Cell (Ballard Power Systems) |
Proton Exchange Membrane (PEM) Fuel Cell
The fundamental component of the Ballard® fuel cell
consists of two electrodes, the anode and the cathode, separated by a polymer membrane
electrolyte. Each of the electrodes is coated on one side with a thin platinum catalyst
layer. The electrodes, catalyst and membrane together form the membrane electrode
assembly.
Hydrogen fuel dissociates into free electrons and protons (positive hydrogen ions) in the
presence of the platinum catalyst at the anode. The free electrons are conducted in the
form of usable electric current through the external circuit. The protons migrate through
the membrane electrolyte to the cathode. At the cathode, oxygen from air, electrons from
the external circuit and protons combine to form pure water and heat. Individual fuel
cells produce about 0.6 Volt and are combined into a fuel cell stack to provide the amount
of electrical power required.
| . | 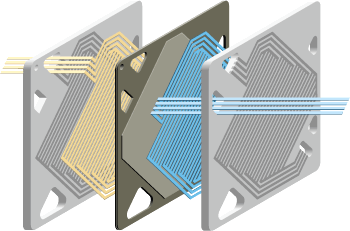 |
| Above: An illustration of the Ballard Fuel Cell (Ballard Power Systems) |
Parts of a Ballard fuel cell
A single fuel cell consists of a membrane electrode
assembly and two flow field plates. Single cells are combined into a fuel cell stack
to produce the desired level of electrical power.
Each membrane electrode assembly consists of two electrodes (anode and cathode) with a
thin layer of catalyst, bonded to either side of a proton exchange membrane (PEM).
Gases (hydrogen and air) are supplied to the electrodes on either side of the PEM through
channels formed in the flow field plates. Hydrogen flows through the channels to the anode
where the platinum catalyst promotes its separation into protons and electrons. On the
opposite side of the PEM, air flows through the channels to the cathode where oxygen in
the air attracts the hydrogen protons through the PEM.
The electrons are captured as useful electricity through an external circuit and combine with the protons and oxygen to produce water vapor on the cathode side.
Reprinted courtesy of Ballard Power Systems